Direct Dye Definition, Chemistry, Applications, Classification and Properties
 |
| Direct Dye |
What is Direct Dye in Textile?
Direct cotton dyes have inherent substantivity for cotton and for other cellulosic fibers. Their aqueous solution dye cotton is usually in the presence of an electrolyte such as NaCl, and Na2SO4. Direct dyes do not require the use of a mordant and as their name implies, the dyeing procedure is quite simple. The goods go into the bath followed by the dissolved dyes. The bath is then gradually heated, usually to a boil, and the addition of salt promotes dyeing
Many direct dyes are relatively inexpensive. They are available in a full range of hues but are not noted for their color brilliance. Their major drawback is their poor to moderate fastness of washing. This limits their use of materials where good washing fastness is not critical. The light fastness of dyeing with direct dyes on cellulosic fiber varies from poor to fairly good, although some copper complex direct dyes have very good light fastness. As usual, the deeper the color of the dyeing, the lower the fastness of wet treatments and the higher the fastness to light. Various Aftertreatments of the dyeings improve the fastness of washing. In some cases, however, aftertreatments can decrease the light fastness. They also invariably cause a change in hue that makes shade correction and color matching more difficult.
Cotton and other cellulosic fibers are dyed with direct, sulfur, vat, reactive , or azoic dyes- more types than for any other fiber. Each of these dye classes has its own application methods, dyeing characteristics, cost, fastness properties, and color range. Therefore, its particular advantages and disadvantages. Within each group, application and performance properties vary considerably. So, the choice of which dyes to use is often not easy. Direct dyes generally cannot meet today's more stringent washing fastness requirements for apparel and linens. In recent years, their share of the market has gradually declined in favor of reactive dyes The latter have very good washing fastness on cellulosic materials and often have bright colors.
Chemistry of Direct Dyes
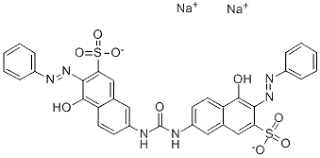
Sulphonated azo dyes constitute the predominant group of direct dyes. These are usually bis, tris, or tetra-zo compounds, the latter type often being brown and black. Direct dyes have usually long, columnar molecular structures.
In general, the greater the extent of conjugation, the longer the wavelength of maximum light absorption. Green dyes must have two absorption bands in the red and violet regions of the visible spectrum. Green poly azo direct dyes, however, tend to be dull and bluish in hue. Bright green direct dyes have blue and yellow dye structures bonded together by a linking group that prevents their mutual conjugation.
 | |
Direct Dye
|
In general, the greater the extent of conjugation, the longer the wavelength of maximum light absorption. Green dyes must have two absorption bands in the red and violet regions of the visible spectrum. Green poly azo direct dyes, however, tend to be dull and bluish in hue. Bright green direct dyes have blue and yellow dye structures bonded together by a linking group that prevents their mutual conjugation.
There are some yellow and orange stilbene direct dyes obtained from the condensation reaction of 4-toluene 2-sulphonic acid. These are often of unknown constitution but have stilbene, azo, and azoxy groups. Sulphonated copper phthalocyanine gives turquoise direct dye. These have good light fastness, but low wet fastness and poor color build-up. Several blue dyes based on the triphenodioxazine structure have good fastness to light. Some metalized azo copper complexes give dyeing of very good light fastness. Many of the older azo direct dyes based on benzidine and its derivatives such as Congo red, and some from 2-naphthyl amine. Direct dyes manufacturing process is no longer manufactured in many countries. Benzidine and 2-naphthyl amine are proven carcinogens.
Although direct dyes structure similar to acid dyes, they generally have higher molecular weights and extended coplanar molecular structures. There is, however, no clear demarcation between acid and direct dyes. Some direct dyes dye protein and nylon fibers as a few acid dyes will also dye cotton.
Application / Uses of Direct Dyes
- Used for dyeing of low price cotton, viscose fabric, curtain, furnishing or carpet with good light fastness and moderate wash fastness
- Cheap cotton dressing gowns and bed spreads which are not washed regularly
- Due to low wash fastness, limited use
- Being replaced to great extent with reactive dyes which have better wash fastnes and have brighter shades
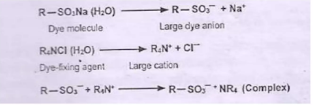
After-treatment of Direct Dye Material
To improve the fastness property
- By increasing the molecular weight and thus decreasing the solubility in water after dyeing
- Cannot be applicable for all the dyes as color of the final product changes
- Treatment with metal salts (Copper salts and chromium compounds): The dyed goods are treated with a bath containing 1. Potassium dichromate (K2Cr2O7) - (2-3)% o.w.f and acetic acid (2-5)% o.w.f . The treatment is for 30 minutes at the boil. The goods are then rinsed and dried
- Treatment with formaldehyde (2-3)% o.w.f, acetic acid 1% o.w.f . The treatment is carried out for 30 min at 60 C followed by rinsing and dyeing
- Treatment with cationic dye fixing agent
 |
| Chmical reaction of aftertreatment |
Stripping
In case of uneven dyeing, patchy dyeing, and also when the shade is unacceptably deeper than desired, the dyes may be required to be stripped
Almost all dyes can be stripped with a hot, dilute solution of caustic soda and sodium hydrosulphite (Na2S2O4)
However, it may be not possible to strip all dyes to a complete white, but the treatment may be good enough for the correction of faulty dyeing
Properties of Direct Dye
Direct dye have some characteristics. Main properties of direct dyes are given below
- Direct dyes are water soluble dyes
- It is anionic in nature
- It need electrolyte for exhaustion
- Dyeing process is carried out in alkaline condition
- Generally applied for cellulosic as well as protein fibers
- Fastness properties are improved by after treatment
- It is widely used as compared with reactive dyes
- Comparatively cheap in price
- Direct dyes are used for cheap goods of local market
Fastness Properties of Direct Dye
- Poor wash fastness due to small dye particles
- Have moderate to good rubbing fastness
- Good lightfastness
- Poor chemical wash fastness
Trade Name of Direct Dye
- Yellow 2RLS
- Yellow R
- Yellow GL
- Brown 2RL
- Orange G
- Orange RL
- Fire Red GLS
- Fire Red G
- Pink 5BLG
- Red 2BL
- Blue 2GLN
- Blue FLE
- Black RE
- Black RL
Mechanism of Direct Dye
When cotton is immersed in a solution of a direct dye the following mechanism take place:
1. Absorption
- Dye molecules move towards the fiber
- Get absorbed on the fiber surface
2. Absorption or penetration
- Absorbed dye penetrate inside the fiber structure
- Gradually penetrate or diffuse inside the structure
- Rate of penetration depends on the molecular structure of the dye and dyeing condition
- Greater the penetration of the dye in the fiber, better and brighter is the dyeing
3. Fixation
- Fixation take place by means of hydrogen bonds and vandarwalls force of attraction
Types / Classification of Direct Dyes
Direct dyes classification according to their chemical structures is not of much use to the dyes since dyes with similar constitutions can have quite different applications and fastness properties. Direct dyes vary widely in their dyeing behavior giving compatibility problems. Grouping direct dyes according to their dyeing properties is therefore more useful. The common classification of direct dyes is that of the Society of Colorists and Dyers., based on their leveling ability and their response to increases in the dyeing temperature and to added salt during exhaust dyeing.
- SDC Class A direct dyes: There are self-leveling dyes with good migration, even in the presence of salt. They usually require considerable amounts of salt for good exhaustion because of their lower substantivity. These dyes are relatively low molecular weight mono and bis-azo dyes with several anionic sulphonate groups per molecule. They therefore have good water solubility and do not aggregate to a significant degree in solution. Dyeing is started at 50 C in the presence of added salt, The bath is heated to a boil for 30-40 min and dyeing continues at a boil for up to an hour. Several further salt additions, of increasing size, are required to promote exhaustion, the total amount of salt depending upon the depth of shade and the liquor ratio. Although these dyes would give greater exhaustion by dyeing at lower temperatures, dyeing at the boil allows good leveling and adequate penetration of the dyes into the fiber.
- SDC Class B direct dyes: These are salt-sensitive or salt-controllable dyes, with poor leveling characteristics. They are of higher molecular weight than class A dyes, often bis and tris azo dyes with just a few sulphonate groups per molecule. They have low to moderate substantivity in the absence of salt but give much-increased exhaustion on the addition of salts to the dye bath. The direct dyeing process is the same as Class A dyes but the initial salt is omitted. The gradual addition of dissolved salt, at the boil, controls the exhaustion
- SDC Class C dyes: These very salt-sensitive dyes exhibit poor migration. Level dyeing depends on the gradual increase of the dyeing temperatures and subsequent additions of the limited amount of salts. Leveling agents may be required. These dyes are temperature-controllable. Dyeing is started at a low temperature without added salt. The bath is slowly heated, with particular care in the temperature region where exhaustion is most rapid. Some salt may be added during further dyeing at the boil. These dyes are often polyazo dyes with few sulphonate groups and are of high substantivity for cellulose. At lower dyeing temperatures, they are very prone to aggregation in solution and sensitive to salt addition. Salt in the dye bath impedes exhaustion at low temperatures because it promotes even more aggregation. The higher the degree of aggregation of the dye, the lower the concentration of individual dye molecules in the solution that can diffuse into the fiber, and therefore the lower the rate of dyeing. Dye aggregates are too large to penetrate into the pores of cellulosic
The SDC classification tests involve dyeing trials using a 30:1 liquor ratio. A migration test and a salt sensitivity test are carried out using the dyes under examination along with standard dyes for comparison. The migration test is conducted at a boil in the presence of 10 % salt using dyeing of the test dye along with an equal weight of undyed cotton. Dyed that exhibit good migration, comparable to that of the standard dyes, belong to Class A. For the other dyes showing poor migration ability, a salt sensitivity test is carried out. Dyeing is prepared at a boil in the presence of 0.6, 0.8, and 1.0% of salt for 30 min. The dyed samples are removed and replaced with the same weight of undyed material, further salt is added to give a total of 20 % of NaCl and dyeing continued for another 30 min. For Class B dys, the initially removed samples, dyed at low salt concentrations, are lighter or the same as the second sample dyed in the presence of additional salt. For Class C dyes, the initial dyeings are darker than the later ones.
An examination of the molecular structures of direct dyes from the three groups shows that in passing from Group A to Group C, dye substantivity generally increases because of increased molecular weight and a lower number of sulphonate groups per dye molecule. Class C dyes therefore generally have better-washing fastness than Class B dyes, with Class A dyes having even lower fastness.
Despite its value, the SDC classification is not readily applicable to dyeing at low liquor ratios as in jet, jig, or pad dyeing. In these cases, a strike test may be a more useful indicator for selecting compatible dyes. At low liquor ratios, dye aggregation is more pronounced since the dye solution is more concentrated for a given % dye. SDC Class B direct dyes may then require temperature control as well as salt control to obtain level dyeing.
Dyeing Method of Direct Dye
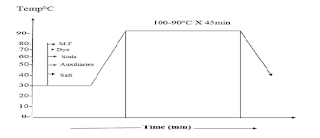
The selection of specific direct dyes for dyeing cellulose fibers depends on their dyeing properties, the particular fastness requirements, any after-treatments used to improve the washing fastness, and the particular finishing process involved. Possible staining of other fibers present in the material by the direct dyes is another consideration. Dyeing with direct dyes is carried out in a neutral solution. The dyebath is gradually heated to promote diffusion and leveling of the dyes, usually with gradual salt addition to exhaust the dyebath
Dyeing direct dye recipe liquor ratios range from about 30:1 for open loose fiber machines, through 20:1 for winch dyeing, 5:1 for jet and package machines, to less than 1:1 in jig dyeing. and padding. Direct dyes usually have good water solubility but the solubility is lower in the presence of salt. At low liquor ratios and temperatures, limited dye solubility may become critical when dyeing deep shades. Any undissolved particles of dye that touch the fabric surface will usually give a dark-colored spot. The dye solution is prepared by pouring boiling water onto an aqueous paste of the dye, prepared with some wetting agent if the powder is difficult to wet. Some dyes require soft water to avoid precipitation of the calcium and magnesium salts of the sulfonated dyes. Others give salts or complexes of different hues with metals such as iron and copper that are sometimes present in the water supply. A sequestering agent such as EDTA will bind unwanted metal ions and prevent the above problems. Sodium hexa meta phosphate is the preferred sequestrant when using copper complex dyes. Agents such as EDTA copper complex dye give a very stable EDTA copper complex and free the non-metalized dye that usually has a different hue.
 |
| dyeing curve of direct dye |
Leveling agents are useful when using dyes that do not easily migrate. These are surfactants, or mixtures of surfactants, based on non-ionic polyethylene oxides and may also contain cationic agents. These chemicals form complexes with many types of anionic dyes, including direct dyes. Complex formation at relatively low temperatures reduces the concentration of the free dye in the bath so that the rate of dyeing is reduced. The dye auxiliary complex molecule is too large to penetrate into the fiber pores. The surfactants in these products disperse the dye-auxiliary complex and prevent its precipitations. As the dyeing temperature increases, the dye-auxiliary complex gradually breaks up, liberating more free dye, which is then available for dyeing. In this way, the rate of temperature increase controls the rate of exhaustion, giving dyeing with improved levelness.
High dyeing temperatures for cellulosic fibers give much better dye migration, a smaller risk of unlevelness, and better fiber penetration. The SDC class B and C dyes migrate better at 120 C, although the dyebath exhaustion is usually lower at this temperature. Decreasing the dyeing temperature towards the end of the process, once the dyeing is level, will then improve the exhaustion. At 120 C, therefore, there is a high risk of decomposition or azo dye reduction by the cellulose. Dyes must be selected with care.
Scouring and bleaching of cotton consume large amounts of steam and hot water. Because of increasing energy costs, many dyehouses dyed grey cotton and cotton/polyester fabric in a combined scouring and dyeing process, providing satisfactory results. This is possible for dull and deep shades on some knitted materials, where the major contaminant to be removed is the lubrication oil used in knitting. For a brighter hue, it is usual to simultaneously scour and dye the goods and then add hydrogen peroxide and alkali to the exhausted dye bath and bleach at 80- 90 C. This is called post-bleaching. In this way, the bath is heated only once, minimizing steam consumption. Dye suppliers recommend dye for these types of combined processes that are stable to alkali and resist fading during post-bleaching.
Problems with tailing arise in padding with a solution of direct dyes because of the inherent substantivity of these dyes for cellulose. To minimize this, the selected dyes should have low, comparable strike rates. Padding may be carried out at higher temperatures in the range of 50-90 C, with a dye solution containing a minimum of salt, to reduce the substantivity. SDC class A direct dyes tend to migrate during drying and steaming and Class C dyes have rapid strike and give most tailing. Class B dyes are therefore often the best choice for continuous dyeing. Dyes of widely differing substantivity obviously cannot be applied together by padding.
The major pad dyeing processes are :
- Pad with dye solution, dry, pad with salt solution, steam
- Pad with dye solution steam
- Pad with salt solution, pad wet-on-wet with dye solution, steam
Cotton/polyester materials can be continuously dyed by padding with a combination of direct and dispersed dyes.
Stripping of dyeing with direct dyes is possible to be hypochlorite or dithionite bleaching, which destroys the dye on the fiber by oxidation or reduction, respectively. This is more difficult with formic acid since it influences re-dyeing. Copper complexes can be demetallized by treatment with EDTA, usually giving a paler shade, and then stripped or redyed
Problems in Batch Dyeing with Direct Dyes
Several problems can complicate the dyeing of cellulosic fibers with direct dyes. The residual chlorine in the fabric from hypochlorite bleaching may bleach some dyes and an antichlor treatment of the goods, reducing the chlorine with sodium bisulfite, then useful before dyeing. Corrosion of the steel in the dyeing machine by sodium chlorite is a constant concern. This is worse at higher dyeing temperatures and then the more expensive Salts or calcine sodium sulfate will be preferred. Half the weight of anhydrous salt is sufficient.
Longer dyeing times and higher temperatures increase the risk of reduction of some azo-direct dyes by reducing groups in the cellulose. This is more likely to occur with the lower molecular weight viscose fibers under alkaline conditions. Ammonium sulfate in the dye bath acts as a slightly acidic buffer to prevent this type of reduction. Alternately, azo dyes reduction by the cellulose can be nitrobenzene sulphonic acids. Dyeing with direct dyes is carried out in a neutral solution. It is necessary to carefully neutralize the goods after alkaline scouring and bleaching and to know the pH of the water supply. Some direct dyes are not stable at high dyeing temperatures and labile groups, such as amide groups as in Cl direct red 83, may hydrolyze.
Rates of Dyeing with Direct Dyes and Compatibility
The rate of dyeing cotton with direct dyes can vary widely from dye to dye. Times of half dyeing of viscose with direct dyes varied by 2-3 orders of magnitude when determined for dyeings in which the final exhaustion was limited to 50% by changing the concentration of added salt in each case. Fortunately, under practical dyeing conditions, given economic exhaustion, dyeing rates do not vary by more than ten-fold.
Rates of dyeing with single direct dyes are very dependent on the actual dyeing condition and may not be a good guide to their properties in the typical dye as a homogeneous dye. As dyeing proceeds, the color of the goods will gradually become deeper but will then always be of the same hue. For this, the dyes in the mixture must have a similar rate of exhaustion and are said to be compatible. The compatibility of the direct dyes depends on the rates of dyeing and migration and the salt sensitivity of the dyes. Hue differences as dyeing proceeds are much more apparent than depth differences. Incompatible dyes also tend to give unlevel dyeings. They have different rates of migration, different degrees of fiber penetration because of dissimilar diffusion rates, and give a change of fabric hue during dyeing because of differences in their overall rates of absorption.
It is preferable to select compatible dye combinations from within each SDC classification group. A combination of some group A, B, and C dyes is possible, however, because of similarities in their properties. Ideally, all the selected dyes should have similar rates of exhaustion and often have the same molecular ionic charge. Dyes with a low rate of exhaustion but good migration are useful for shading towards the end of the dyeing process.
A dip test is useful for establishing dye compatibility. Small pieces of cotton of equal weight are dyed in the same bath with a mixture of direct dyes. At various intervals, a small dyed sample is removed from the bath and replaced by an identical piece of undyed fabric. A series of dyed samples arranged in order of increasing dyeing time will have gradual dyeing color depth, but the invariant hue, when the dyes used are compatible. When this is so, the series
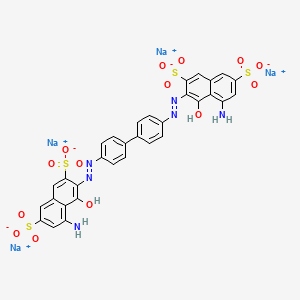
of samples that were added to the dyebath during dyeing will be of increasing color depth, but also with the same constant hue, when arranged in of their times. The results of the compatibility test for direct dyes are only valid under the given dyeing conditions. Rates of exhaustion are very dependent upon the dyeing temperatures and salt concentration. For example,, for a mixture of Cl direct dyes, cotton more rapidly than the blue dye in 0.01 M NaCl, but less rapidly in 0.10 M NaCl.
For some mixtures of two direct dyes, the presence of the second dye reduces
 |
| Cl Direct Blue |
THE EFFECTS OF VARIATIONS IN DYEING CONDITIONS
The influence of added salt
The gradual addition of salt to the dye bath assists in direct dye exhaustion onto cellulosic fibers. The actual effects of this on the rate of dyeing vary considerably from dye to dye the salt provides sous ions to counteract the negative surface potential of de-set cotton All Gbres immersed in water develop a negative surface potential & characterize any two different phases its contact - one will develop an electric charge at its surface opposite in sign to the change of the other This cur because one surface is a better electron acceptor than the e in the case of cellulose at direct dye pH 7, water is a better electron donor than the Negatively charged carboxylate groups, from oxidation of the primary alcohol group on carbon in some glucose units, also augment the surface charge The negative charge of the cellulose surface repels anionic dye molecules.
A high concentration of wounding chum ions counteracts this. Rapid solution Awal helps to break down the surface laver in which this change is effective. Mest measurement of the potential difference between the inside of the fiber. where the dve i best, and the external solution is impossible. Streaming or perennials, which develop when aqueous solutions flow across a fiber plus holl between electrodes, can be measured. The values are probably lower than the el potential difference between the inside of the bee and the external solution As expected, the negative zeta potential of cotton is reduced by salt solutions, Bechtle those containing polyvalent metal ions, and increased by adsorption of direct dyes
The science of added salt is the most important factor in the dyeing of diglossic Abees with direct dyes. In dyeing, there are two objectives good akustion and good colour uniformity. Both depend on the salt controllability of the dyes, or how the gradual salt additions during dyeing influence the rate of hton. Unfortunately, salt controllability is very dependent on the dyeing ensure For practical purposes, it is useful to have some idea of the degree of shaustion at different salt concentrations for each dye, possibly at different heng temperatures. For two dves having about the same substantivity, salt should have a greater influence on the exhaustion of the one with the larger number of phonate groups, and therefore the larger negative molecular charge. The fire's gitive nitface potential repels the more highly charged dye molecules to a prater extent so this dye will respond more to salt additions. This conclusion however is not necessarily of great practical value because commercial direct dyes contain many electrolytes
The nature of the anion of the added electrolyte has little influence on the amount of adsorbed dye. Therefore, NaCl and Na SO, at the same total sodium concentration, have about the same influence. A higher positive charge on the non promotes increased adsorption because polyvalent metal ions counteract the negative surface potential more effectively, thus decreasing the repulsion of approaching dye anions. Polyvalent metal salts are, however, much more expensive than sodium chloride, the usual choice of the dyer.
The effect of temperature
Increasing temperature increases the rate of direct dyeing mechanism and dye migration. From Le Chatelier's principle for an exothermic dyeing equilibrium, the exhaustion will decrease as the dyeing temperature increases, as for some direct dyes on cellulosic fibers. Despite this, higher dyeing temperatures ensure good leveling and better penetration of the dye into the fibers in tightly packed yarns. In practice, such direct dyes are often allowed to finally exhaust in a cooling bath. For isothermal dyeings at increasing temperatures, some dyes, however, show an initial increase in equilibrium exhaustion, up to a temperature of maximum exhaustion, after which the exhaustion then decreases again as the dyeing temperature increases more. Still others, exhibit only increasing exhaustion at temperatures up to the boil.
These diverse variations of direct dye exhaustion depend on two opposing influences of the increasing dyeing temperature. These are the usual effects of
increasing temperature decreases the dyebath exhaustion because dyeing is exothermic, and it enhances the dyeing rate, particularly at lower temperatures. Increasing temperature also promotes dye de-aggregation in the dyeing solution liberating more individual dye molecules to enter the fibre..
Manufacturers often cite a temperature of maximum exhaustion and provide optimum dyeing temperature profiles. The dyes CI Direct Yellow 12, Direct Red 81, and Direct Yellow 28 have maximum exhaustion at 30 °C, 60 °C, and 100 °C, respectively, corresponding approximately to the behavior of dyes (a), (b), and (c). Despite this, the actual dyeing temperature may often depend on machinery limitations. For example, open jig dyeing machines cannot achieve dyeing temperatures much above 85 °C.
The Influence of Direct Dyes Dyeing pH
Dyeing with direct dyes is usually carried out in a neutral solution. Under alkaline conditions, cellulose fibers have an even greater negative potential, partly because of the increasing dissociation of several cellulose hydroxyl groups, and exhaustion is lower. Oxycellulose, present in cotton that has been over-bleached, has a higher proportion of carboxyl groups and is dyed much paler than undamaged cellulose because carboxylate ions repel the dye anions of like charge. Dyeing in the presence of formic acid suppresses the ionization of the carboxyl groups and any cellulose is then dyed to about the same depth of shade as regular cellulose. Under alkaline conditions, reducing end groups, particularly prominent in low molecular weight celluloses such as viscose, reduce some azo direct dyes, decreasing the color yield. In general, however, if the dyeing of cellulosic fibers is in an acidic solution, only weak acids are used and the residual acid in the goods is thoroughly rinsed out before drying. Strongly acidic dyeing conditions favor the acid-catalyzed hydrolysis of cellulose. Any traces of residual mineral acid dried into the material can cause considerable damage.
The Effect of Liquor Ratio
As we saw in Section 10.4.3, dyebath exhaustion should increase with a decrease in the dyeing liquor ratio. This is certainly the case for direct dyes. This means that dyeing at a low liquor ratio decreases the amount of waste dye in the effluent. It also consumes less water and steam and allows a given salt concentration with less added salt. There has been a strong trend towards dyeing at as low a liquor ratio as practicable. It should always be remembered, however, that the required amount of dye must then be dissolved in a bath of reduced volume. It is important to ensure that the dye is, in fact, in solution at the beginning of dyeing when the temperature is relatively low and after the addition of salt.
THE AFTER-TREATMENT OF DYEINGS WITH DIRECT DYES
Treatment of dyeings of direct dyes on cellulosic materials aims to improve the washing fastness by increasing the dye's molecular weight. This makes it less soluble and of slower diffusion. Some of these processes decrease the light fastness of the dyeing. Aftertreatments are difficult and costly to carry out and often give changes in hue that greatly impede shade correction and color matching. Many of the direct dyes after treatments now have limited use because direct dye vs reactive dyes give dyeings much better washing fastness.
Diazotisation and Development
Diazotization of direct dyes with primary aromatic amino groups, followed by coupling of the diazonium ion with an appropriate developer, can be very effective after treatment. Primuline, CI Direct Yellow 59 (7, in Figure 14.5), is a classic direct dye examples of such a dye. Diazotization involves treating the yellow dyeing with an acidic solution of sodium nitrite at room temperature or lower. Both amine and phenol developers can be used. The diazotized dye in the material is sensitive to light and heat so immediate coupling in a second bath containing the developer is necessary. A final wash removes any dye deposited on the fabric surface to ensure good fastness to washing and rubbing. Although the washing fastness improves by about one grade, there is often a considerable change in hue. For example, dyeing with Primuline, diazotized, and developed with 2-naphthol (8), turns from yellow to red. This technique is useful for cheap navies and blacks with a washing fastness of 3-4 but is little used today.
Coupling with Diazonium Salts
Direct dyes with free positions ortho and para to hydroxyl or amino groups react with an appropriate diazonium ion to introduce one or two additional azo groups P-Nitroaniline, diazotised in a freshly precipitated suspension of its hydrochloride, has been widely used for this purpose.
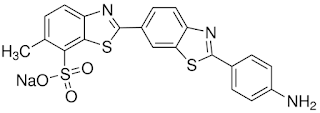 |
| Primuline Cl Direct Yellow |
After treatment with Formaldehyde
This type of after-treatment applies to a few mostly black dyes. Dye molecules are linked by methylene groups, usually in ortho positions to hydroxyl or amino groups. The dyeing is treated with acetic acid and formaldehyde in solution at 70- 80 °C. This after treatment may result in decreased light fastness.
Metal Complex Formation
In this type of aftertreatment, cupric or chromic ions convert the dye into a metal complex. The most common ligand structure is an o,o'-dihydroxyazo compound, or its dimethyl ether. In the latter case, the methyl groups are displaced and the dye forms the complex of a dihydroxyazo compound (9 and 10). Coppering involves treating the dyeing with acetic acid and copper sulfate solution and heating it to 70-80 °C. Complexation usually increases the light fastness. The process results in a change in hue and may be reversible on repeated washing due to de-metallization. This results in a gradual decrease in washing fastness. Both copper and chromium are environmentally undesirable. Copper treatment is still used for a few brown, navy, and black shades, taking care that
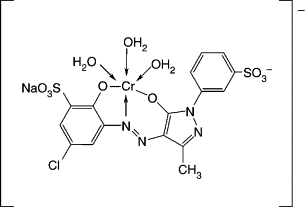 |
| Metal Complex Formation of Direct Dye |
there is a minimum of copper ion in the effluent. Because of this problem, most dye-copper complex dyes are pre-metalized by dye manufacturers. Even the release in the effluent of small amounts of copper in unexhausted p metalized direct dyes is now reaching the point of exceeding allowable limits. pre-
Cationic Fixatives
This after-treatment involves the precipitation of anionic dyes in the cotton with a cationic surfactant or polymer in warm water. It tends to reduce the fastness of light of the dying. The change in hue is only slight
Resin and Crosslinking Agents
Amino resins and crosslinking agents impart dimensional stability to cotton and viscose fabrics and provide crease resistance and easy-care direct dye properties. These finishes also improve the wet fastness of direct dyes pdf but again may decrease the light fastness and give a hue change.
The Indosol SF range of dyes (Clariant) are SDC Class B and C copper complex direct dyes. After dyeing, the cotton fabric can be finished by padding with Indosol CR liquid, followed by drying and curing. This imparts dimension stability and crease recovery as well as good washing fastness and moderate to good light fastness.
DYEING DIFFERENT TYPES OF CELLULOSIC FIBRES
Cellulosic fibers include cotton, mercerized cotton, linen, and various forms of rocks, each with its own particular dyeing characteristics. Because of the differences in molecular weight and morphology between native and regenerated cellulose fibers, there are large differences in their physical properties. Wet cotton is a strong, rigid fiber and most woven cotton fabrics will withstand considerable stress. This is not true of fabrics made from regular viscose. Their wet strength is only moderate and they require careful handling during dyeing. Viscose also swells much more than cotton when wetted. This can lead to limited solution flow and dye penetration problems in dyeing packages or packed loose fiber.
The different types of cellulosic fiber are all dyed by direct dyes using essentially the same dyeing method. The temperature increase of the dyebath, and the amount of salt added to it. control the rate of exhaustion and leveling. In the dyeing of blends of these cellulose fibers with direct dyes, they do not absorb dyes at the same rate or to the same extent because of the differences in their morphology. Even the extent and conditions of drying after preparation can influence the dyeing of a fabric of cellulosic fibers. Despite these differences, the standard affinity of a given direct dye in textile is remarkably constant for the different cellulose fibers.
The effects of carboxylate groups are important at low salt concentrations where there is little effective shielding of their negative charge by sodium ions. The higher negative surface charge of viscose compared with cotton is partly because of its additional carboxylate groups. In general, with more than 2 g NaCl, the extent of dye absorption increases in the order cotton, mercerized cotton, and regular viscose; in the same order as increasing fiber accessibility. Even different grades of cotton fiber have different dye absorption characteristics. This is one reason why the intimate blending of cotton fibers before spinning is so important.
The rate of dyeing increases as the diameter of a fiber decreases, even though the equilibrium exhaustions for chemically identical fibers with different diameters hardly vary. This is readily demonstrated for viscose filaments of different deniers The internal volume of the pores is different for the various cellulosic fibers. These internal volumes have been estimated to be 0.30, 0.50, and 0.45 1 kg for cotton, mercerized cotton, and regular viscose. A value of 0.22 1 kg is widely used for cotton in estimating dye affinities (Section 11.1.2). In dyeing, these fibers are far from being saturated with dye, so the dye only adsorbs onto a very small part of the internal surface.
Covering neps of immature cotton fibers is always a concern in internal surface. cotton dyeing. With dyes of low substantivity, reps usually absorb less dye and appear as paler spots on the fabric. Since immature fibers give much greater tales of dye desorption, the paler dyed neps may not appear until after washing Bees coverage can be achieved using dyes of high substantivity and can be m improved by mercerisation before dyeing, since this swells the immature fees (Section 5.4.6).
Mercerized cotton will absorb more dye and be darker in color than form cotton dyed in the same bath. It is more accessible than regular cotton and its lower negative surface potential decreases the repulsion of dye anions. It will appear darker in color even for the same amount of dye in the fiber because of a degree of internal light scattering that results in greater light absorption. This effect is, however, dependent on the dye being used and the conditions of mercerisation.
Viscose fibers and filaments are available in several variants with different dyeing characteristics. They have different internal structures, skin-to-core ratio porosities, and degrees of fibrillation. These different forms therefore have different dyeing rates, equilibrium dye absorptions, and colour yields. Direct dyes have higher substantivity for regular viscose than for cotton because viscose is less crystalline and oriented so it has a much greater internal surface. The fastness to light of dyeings of direct dyes on viscose is up to one fastness grade better than on cotton. The fastness of washing on viscose, however, is often less than on cotton because of its higher accessibility. The more highly oriented viscose types give better-wet fastness.
The physical and chemical properties and the dyeing behavior of the various cellulosic fibers are therefore quite different. About all they have in common, is the cellulose that constitutes them.
THE ORIGINS OF SUBSTANTIVITY FOR CELLULOSE of Direct Dyes
The dyeing of cotton with direct dyes is completely reversible. For dyeing equilibrium, the Freundlich isotherm usually applies but some dyeings with direct dyes give a better correlation with the Langmuir isotherm. The former isotherm usually indicates that adsorption is non-specific and the latter
that the dye adsorbs on specific fiber sites. This dichotomy of behavior is typical of the poor understanding of dyeing cellulose fibers. Direct dyes invariably have extended, conjugated, coplanar molecules, with
widely spaced hydrogen bonding groups and some sulphonate groups to provide solubility in water. The greater the number of sulphonate groups in the dye molecule, the higher the water solubility but the lower the fastness to wet peatments, and the lower the dye substantivity. Molecular coplanarity is, however, a specific requirement for substantivity. Whereas the dye Benzopurpurine 4B (11, in Figure 14.7) has good substantivity for cotton, its isomer meta-Benzopurpurine (12), which cannot be coplanar, does not. While most dyes substantive to cellulose fibers are coplanar, they are not always long and linear (see Chapter 17 on vat dyes).
Long coplanar dye molecules can sit on top of a cellulose polymer chain with the aromatic rings parallel to the glucose rings. This would allow short-range intermolecular attractive forces to operate. This interaction has long been considered to involve hydrogen bonding. Cellulose has an abundance of hydroxyl groups and direct cotton dyes often have hydroxyl, amino, or amide groups capable of hydrogen bonding. This interpretation of the origin of the substantivity of direct dyes is prevalent in the dyeing literature. It is by no means proven. The cellulose hydroxyl groups are in the equatorial positions around the glucose units and do
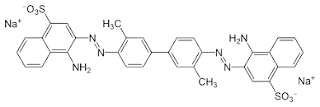 |
| Bezopurpurine of Direct Dye |
not necessarily present an appropriate angle to the hydrogen bonding groups in the dye molecule when the latter is sitting above or below the glucose ring. Cellulose hydroxyl groups are usually hydrogen bonded together or to water during dyeing. Many leuco vat dyes, derived from polycyclic quinones and leuco sulfur dyes (Chapter 17) have high substantivity for cellulose fibers, yet they have neither linear structures nor many hydrogen bonding groups. Substantivity may therefore depend more on van der Waals and dispersion forces between the dye and fibre molecules.
Substantivity may also involve the surface active properties of direct dyes. The more hydrophobic sections of the dye molecule would prefer to approach the relatively hydrophobic axially oriented carbon-hydrogen bonds of the glucose rings in cellulose to avoid interaction with surrounding water molecules. This is the driving force of surface activity. Since cellulosic fibers are extremely porous, they have an extended internal surface and surface activity effects could account for a considerable degree of dye adsorption.
Direct dyes aggregate in solution and in the fiber, another facet of their surface activity. There is circumstantial evidence that aggregation of the dye molecules in the cellulose fiber is the cause of the dye's substantivity. Dye aggregation increases with increasing dye and salt concentration, and decreases with increasing temperature. Most evidence suggests that aggregation is not significant in boiling solutions but the inability of the limited experimental techniques to detect small aggregates in the solution, or in the fiber, could give misleading conclusions. Some dyes do not appear to aggregate but are substantive (CI Direct Yellow 12). Others are so highly aggregated that dyeing is almost impossible at low temperatures (copper phthalocyanine direct dyes), and others appear to have no substantivity unless salt is present (CI Direct Blue 1).
Despite the considerable literature on the effects of varying molecular structures on the substantivity of dyes for cotton, the origins of such substantivity are poorly understood. Are both dye-fibre and dye-dye interactions as well as surface activity involved in promoting substantivity of direct dyes for cellulosic fibres? The best answer one can give is possible. How little do we understand this technology?
F.A.Q
REFERENCES
1. J. Shore, Cellulrisics Dyeing, J. Shore. Ed (Bradford: SDC, 1995).
2. SDC Committee on Direct Dyes. J.S.D.C., 62 (1946) 280, 64 (1948) 145.



Comments
Post a Comment